A lot of companies will purchase a pre-made “canned” quality system when they start developing medical devices. Proponents of this approach argue that the system itself has been proven in regular audits by multiple companies and, as such, they are an ideal place to start. The reality is that these generic systems are compliant only because they impose extensive “commercial-ready” controls. They attempt to cover every possibility without making any distinctions about the development stage, product type or internal company processes. Furthermore, because they are so generic they are virtually unusable in the as-purchased state. Finally they are expensive both to purchase up-front and to operate.
DTG has a different approach. Instead of relying on a pre-designed generic system, we use our experience to help you create and operate a customized system that will keep you in compliance for your current development stage WITHOUT added burdening you with processes that you don’t YET need. This leads to a more efficient system (both time AND money) as it does not impose controls that are unnecessary.
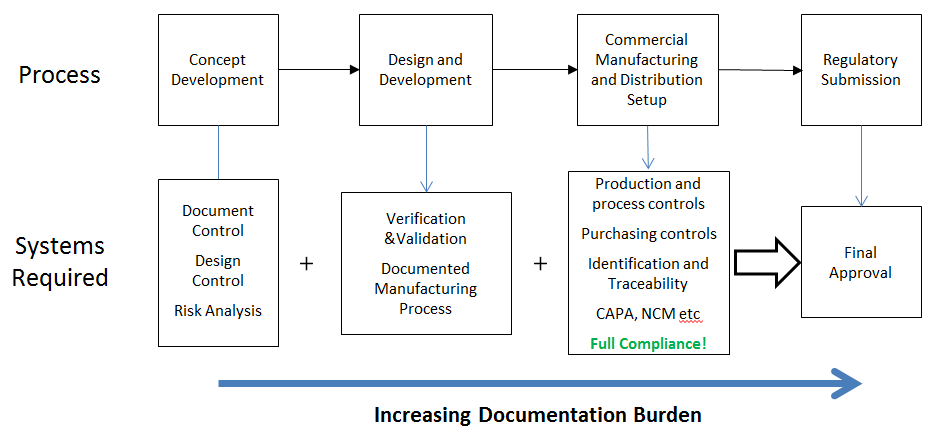
The diagram above illustrates (in a simplistic way) the development path that is expected by ALL of the regulatory bodies regardless of jurisdiction. As you move through development, amount of process control increases and the documentation burden rises. However, when you are developing a new and innovative product it is key that you keep the process as flexible as possible – especially in the early stages. After all, you are here because you are developing something no-one else has done before and you need to be flexible to do that. Implementing commercial manufacturing controls in the design and development stage will slow you down, increase costs and frustrate your engineers without providing any benefit towards the final product. Only by keeping the development stage, product type and company processes in mind can an efficient system be developed and operated.
For a more detailed explanation of the various development stages, see our development process page.
Medical device development isn’t something you should do alone. Contact Us. We can help.